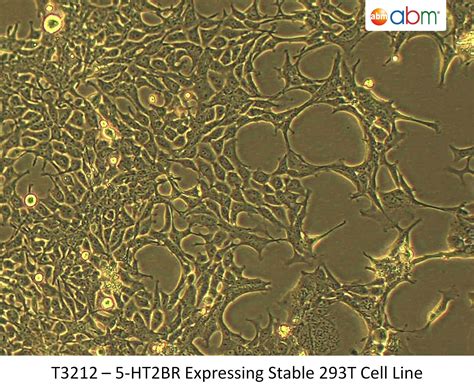
The term 293T Cell Stable Cell refers to a well-characterized, long-lasting cell-line option widely used in biomedical research to study gene expression, protein production, and screening in a controlled context. Understanding what defines stability in a cell line—and why the 293T Cell Stable Cell lineage is a popular choice—helps researchers align expectations with project goals. This article explains the concept of stability, outlines typical attributes of a 293T Cell Stable Cell, and highlights factors to consider when evaluating sources for research use.
Key Points
- Stability in a cell line reflects consistent performance across passages, which the 293T Cell Stable Cell concept emphasizes for reproducible results.
- Quality controls like identity authentication and contamination checks help ensure the reliability of a 293T Cell Stable Cell source.
- These cells offer robust growth and broad compatibility with common research assays, making them a versatile platform for high-level studies.
- Transparency from suppliers—documentation, certificates of analysis, and licensing terms—supports credible usage of a 293T Cell Stable Cell.
- Consider safety, regulatory compliance, and facility readiness when planning projects that involve a 293T Cell Stable Cell.
What is a 293T Cell Stable Cell?
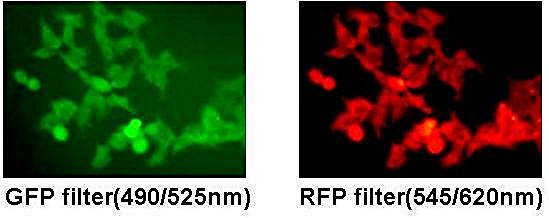
In general terms, a 293T Cell Stable Cell refers to a cell line that maintains stable properties over time, with predictable growth and consistent expression patterns across passages. The 293T lineage, derived from human embryonic kidney cells, is valued for its robustness and ease of handling in many research contexts. A stable variant emphasizes long-term reliability, facilitating comparisons across studies and reducing variability.
Key features of stability
Typical attributes include consistent morphology, steady growth rate, and stable expression profiles for key markers. While actual performance depends on many factors, a 293T Cell Stable Cell generally offers a dependable platform for non-procedural, high-level investigations.
Benefits and applications of the 293T Cell Stable Cell
Researchers often choose this cell type for its adaptability in exploratory research, educational demonstrations, and background studies that require repeatable data. The 293T Cell Stable Cell platform supports wide-ranging applications, from basic biology concepts to translational observations, without tying researchers to specialized protocols.
Common, high-level uses
General use cases include studies of gene expression, protein production at a conceptual level, and model systems for evaluating hypotheses in a controlled cellular context. A stable line helps researchers focus on interpretation rather than variability.
Considerations for selecting a 293T Cell Stable Cell source
Choosing a supplier or repository involves assessing documentation, authenticity, and safety considerations. For a 293T Cell Stable Cell, look for clear strain history, identity verification, contamination screening, and certificates of analysis. Licensing terms and user support can also influence long-term reproducibility.
What to ask a supplier (high-level)
Ask about traceability, passage history, STR profiling, and available quality-control data. The goal is to confirm that the 293T Cell Stable Cell meets your project expectations without disclosing procedural steps for handling.
Storage, handling, and regulatory considerations (high-level)
At a high level, storage and handling guidance should align with institutional biosafety policies and local regulations. Understanding the regulatory landscape helps teams plan responsibly when incorporating a 293T Cell Stable Cell into research plans.
What is a 293T Cell Stable Cell, and how does stability affect research?
+A 293T Cell Stable Cell denotes a line that maintains consistent characteristics across time and passages, contributing to more reliable and comparable results in non-procedural, high-level studies. Stability reduces variability and helps researchers interpret findings with greater confidence.
<div class="faq-item">
<div class="faq-question">
<h3>What are common considerations when evaluating a vendor for a 293T Cell Stable Cell?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Look for clear documentation, authentication data, contamination screening, and certificates of analysis for the <strong>293T Cell Stable Cell</strong> product. Transparent licensing and support quality also influence reproducibility and long-term planning.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Are there safety and regulatory aspects to keep in mind with 293T cell lines?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Yes—consider institutional biosafety requirements and local regulations when working with human-derived lines. While this article stays at a high level, compliance and responsible research practices are essential when using a <strong>293T Cell Stable Cell</strong>.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Where can I learn more about reputable sources for 293T Cell Stable Cell lines?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Consult institutional core facilities, recognized cell banks, and supplier documentation to compare options for a <strong>293T Cell Stable Cell</strong>. Seek reviews of data quality, support, and long-term availability to inform your choice.</p>
</div>
</div>